THE PERIODIC TABLE
Modern periodic table is also called long form of the periodic table or Bohr?s table. In this table, the elements are arranged in order of their increasing atomic number. It consists of 4 blocks (s, p, d and f), 18 groups numbered from 1 to 18 and 7 periods numbered from 1 to 7.
Blocks : The periodic table is divided into four main blocks (s, p, d and f) depending upon the subshell to which the valence electron enters into.
(1) Elements of group 1 and 2 constitute s-Block.
(2) Elements of group 13, 14, 15, 16, 17, 18 constitute p-Block
(3) Elements of group 3, 4, 5, 6, 7, 8, 9, 10, 11, 12 constitute d-Block
(4) The f-Block elements comprise two horizontal rows placed at the bottom of the periodic table to avoid its unnecessary expansion.
Elements of s- and p-blocks are called normal or representative elements, those of d-block are called transition elements while the f-block elements are called inner transition elements.
Groups : The 18 vertical columns are called groups. The elements belonging to a particular group is known as a family and is usually named after the first number. Apart from this some of the groups are given typical names as examplified beneath,
(1) Elements of group 1 are called Alkali-Metals.
(2) Elements of group 2 are called Alkaline Earths.
(3) Elements of group 15 are called Pnicogens.
(4) Elements of group 16 are called Chalcogens.
(5) Elements of group 17 are called Halogens.
(6) Elements of group 18 are called Noble Gases or Aerogens.
All the other groups are named after the first member of each group.
Periods : The horizontal rows are called periods. There are seven periods in the long form of the periodic table,
(1) Ist period
contains 2 elements. It is the shortest period.
(2) 2nd period
and 3rd period
contains 8 elements each. These are short periods.
(3) 4th period
and 5th period
contains 18 elements each. These are long periods.
(4) 6th period
consists of 32 elements and is the longest period.
(5) 7th period starting with
is incomplete and consists of 19 elements.
Well,
The periodic table we use today is based on the one devised and published by Dmitri Mendeleev in 1869.
Mendeleev found he could arrange the 65 elements then known in a grid or table so that each element had:
1. A higher atomic weight than the one on its left. For example, magnesium (atomic weight 24.3) is placed to the right of sodium (atomic weight 23.0):
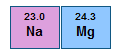
Mendeleev realized that the table in front of him lay at the very heart of chemistry. And more than that, Mendeleev saw that his table was incomplete - there were spaces where elements should be, but no-one had discovered them.
Just as Adams and Le Verrier could be said to have discovered the planet Neptune on paper, Mendeleev could be said to have discovered germanium on paper. He called this new element eka-silicon, after observing a gap in the periodic table between silicon and tin:

Similarly, Mendeleev discovered gallium (eka-aluminum) and scandium (eka-boron) on paper, because he predicted their existence and their properties before their actual discoveries.
Although Mendeleev had made a crucial breakthrough, he made little further progress. With the benefit of hindsight, we know that Mendeleev's periodic table was underpinned by false reasoning. Mendeleev believed, incorrectly, that chemical properties were determined by atomic weight. Of course, this was perfectly reasonable when we consider scientific knowledge in 1869.
In 1869 the electron itself had not been discovered - that happened 27 years later, in 1896.
In fact, it took 44 years for the correct explanation of the regular patterns in Mendeleev's periodic table to be found.
The Explanation is Found
The explanation came in 1913 from Henry Moseley, who fired electrons at atoms, resulting in the emission of x-rays. Moseley found that each element he studied emitted x-rays at a unique frequency.
The explanation came in 1913 from Henry Moseley, who fired electrons at atoms, resulting in the emission of x-rays. Moseley found that each element he studied emitted x-rays at a unique frequency.
When he looked at the frequencies emitted by a series of elements, he found a pattern that was best explained if the positive charge in the nucleus increased by exactly one unit from element to element.
A Graph Summarizing Moseley's Results
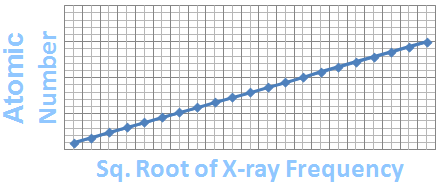
In other words, Moseley had found that elements are different from one another because their atoms have different numbers of protons. He discovered that elements' positions in the periodic table are better predicted by their atomic numbers than their atomic weights. (An element's atomic number is equal to the number of protons, and hence electrons, in one of its atoms.)
Moseley's discovery cleared up the cobalt-nickel and argon-potassium problems.
Considering the argon-potassium problem, it was known that argon has a higher atomic weight than potassium. According to Mendeleev's reasoning, argon should therefore be placed after potassium in the periodic table. But, doing this made no sense in terms of chemical properties.
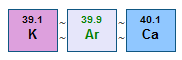
Moseley's work showed that argon's atomic number is 18 and potassium's is 19. Therefore argon should be placed before potassium in a periodic table based on atomic numbers. Chemists around the world breathed a collective sigh of relief, because this agreed with the observed chemical properties of these elements.

Moseley also emulated Mendeleev's achievement of discovering new elements on paper, finding four atomic numbers with no matching elements. He predicted the existence of elements with atomic numbers 43, 61, 72, and 75. These elements were indeed discovered; we now call them technetium, promethium, hafnium and rhenium.
Today the chemical elements are still arranged in order of increasing atomic number (Z) as we look from left to right across the periodic table.
Valence Electrons
It is the valence electrons which cause chemical reactivity.
All of the elements in Group 1 have one valence electron; Group 2, two valence electrons; Group 13, three valence electrons; Group 14, four valence electrons; Group 15, five valence electrons; Group 16, six valence electrons; Group 17, seven valence electrons; and Group 18, eight valence electrons, except for helium, which has two.
Group 18 is the noble gas group, a group of unreactive elements. The reluctance of the noble gases to react chemically is the key that unlocks our understanding of why other elements do react.
The noble gases are unreactive, because their outer electron shells are full. A full shell of outer electrons is a particularly stable arrangement. This means that noble gas atoms neither gain nor lose electrons easily; they react with other atoms with great difficulty, or not at all.
Other atoms lose electrons, gain electrons or share electrons to achieve the same electron configuration as a noble gas - in doing so, they form chemical bonds, and make new substances.
When an atom loses or gains one or more electrons, it can no longer be described as an atom - it is called an ion.
All ions are either positively or negatively charged.

Since our sodium atom has lost a negatively charged electron, it becomes a positively charged sodium ion: Na+. This sodium ion, with one electron fewer than the sodium atom, has the same electron configuration as the noble gas neon and is chemically stable.
The chlorine atom, which starts with seven valence electrons, gains a single electron and becomes a negatively charged chlorine ion: Cl-. This ion has the same electron configuration as the noble gas argon, and therefore this ion is chemically stable too.
The positively charged sodium ion and negatively charged chlorine ion attract one another electrostatically, forming a stable chemical compound, sodium chloride. These electrostatic bonds are called ionic bonds.
For the transition metals the situation is a little more complicated than those described above, because electrons from the lower shells in transition metal atoms can become valence electrons. This is why, for example, we can get different types of copper ions, Cu+ and Cu2+, and iron ions, Fe2+ and Fe3+.
The number of valence electrons in atoms is the basis of the regular patterns observed by Mendeleev in 1869, patterns which ultimately have given us our modern periodic table.
